Precipitated Calcium Carbonate Making process (light powder CaCO3)
Light powder CaCO3 is a white powder, slightly soluble in water, but soluble in most acids. CO2-saturated water also dissolves CaCO3 significantly because CaHCO3 is much more soluble than CaCO3. Light powder has many applications. In the pharmaceutical industry, it is used as an excipient for some tablets with high quality requirements.

Preparation of light powder CaCO3:
Light powder production technology is relatively simple, with a small initial investment, we can actively meet domestic and export demand. To produce light powder, people are based on the following principle: Blow CO2 gas flow. Boiled in the lime milk solution, precipitated and collected CaCO3. The main raw materials for making light powder are quicklime and CO2 that the lime revives.
From limestone ore, The reaction process from calcination to light powder is:
CaCO3 -> CaO + CO2
(ore)
CaO + H2O --> Ca(OH)2
Ca(OH)2 + CO2 --> CaCO3 (precipitate) + H2O
Thus, the light powder production process can be done at the same time as the lime calcination process.
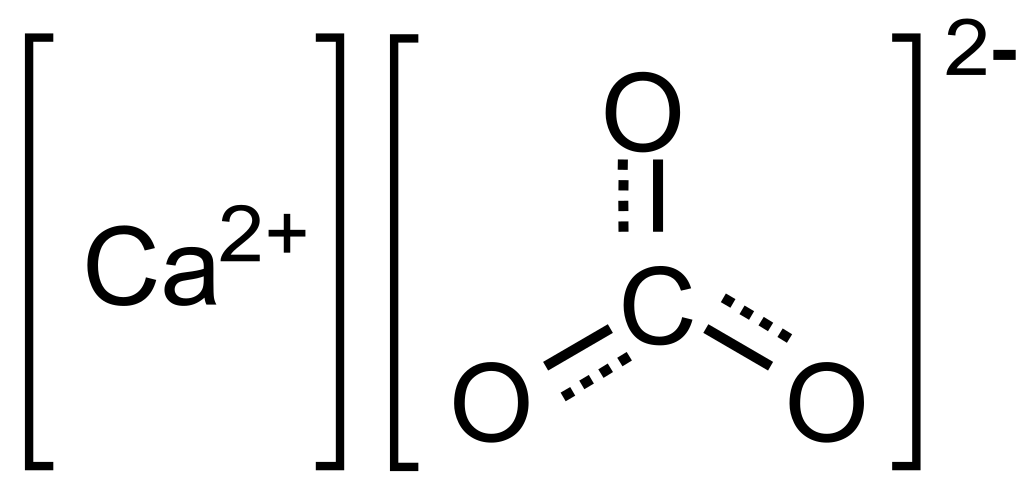
Making lime milk:
Select freshly cooked lime, without any impurities. Putting lime into clean water, stir well, soak for 48 hours for a thorough reaction, and take lime milk about 7-8 Baume degrees. If we put solid lime milk, the reaction will be long and difficult to convert to CaCO3. On the other hand if the lime milk is diluted, the efficiency will be low and uneconomical. Before putting the lime milk into the reaction tank, the pump runs through the hydraulic cyclonic system to remove all solid particles mixed in the lime milk.
CO2 absorption process:
The source of CO2 can be obtained from lime kiln gas. The burned lime kiln gas is no longer emitting black smoke, we collect CO2 by sealing the kiln's mouth with a corrugated iron lid with a cone for CO2 to pass through the duct into the air filter system with continuous irrigation water upstream, then clean the gas and cool it down before entering the reactor. In order to facilitate aeration and reaction, the solution in the reaction tank is continuously stirred by a specialized stirrer.
The higher the CO2 concentration in the reaction tank, the better, usually the lime kiln gas with 20-30% CO2 is a satisfactory reaction, if the CO2 is less than 15%, the reaction is very long and the product is not good, then it is necessary to Add coal and stone to the kiln. Check the reaction solution with 1% alcohol phenolphthalein reagent. After the reagent does not appear pink, we can remove the reaction solution to the filter tank covered with white canvas to retain CaCO3 in the form of a paste. It is advisable not to aerate the CO2 for a long time because then CaCO3 is easily converted to CaHCO3 which dissolves in a considerable amount in water.
Drying:
Before drying, put the paste into the extractor (centrifuge) to remove the water. After centrifugation, the water content is only about 50%, put the powder on the drying rack directly or dry it with hot air in a rotary kiln. The drying temperature is 110 Censius degree, the obtained product has a moisture content of ≤ 0.50%. The dry powder is ground through a hammer mill. Packed in kraft paper (4-5 layers of cement bags), or P.E bags. Stored in specialized, dry and clean warehouses. The quality of light calcium carbonate must meet the following criteria:
+ CaCO3 content ≥ 98%.
+ Alkalinity (calculated as CaO) 0.15%
+ Humidity ≤ 0.50.
+ The content of substances insoluble in hydrochloric acid (HCl) ≤ 0.25.
+ Fineness through sieve 0.125 φ mm ≥ 98.
Our Company has experiences in manufaturing and distributing types of Limestone and Calcium Carbonate.
For any information, please contact:
NO.18 SON HA MINERALS COMPANY
Factory: Mam Xoi Mountain, Thanh Son Commune, Kim Bang District, Ha Nam Province, Viet nam
Telephone: +84 931 717 698 Fax: +84243.5190937
Hotline: +84 936 212 598 (Mr. Thomas) / +84 931 717 698 (Mr. Henry)
Email: export2@shcgroup.vn (Mr. Thomas) // manager.shcgroup@gmail.com (Mr. Henry)





COMMENTS:
bonqltcqe Reply
Precipitated Calcium Carbonate Making (light powder CaCO3) bonqltcqe http://www.gt0256m6w1bofg41v7892ka041dsy6eas.org/ abonqltcqe [url=http://www.gt0256m6w1bofg41v7892ka041dsy6eas.org/]ubonqltcqe[/url]
28/11/2022rihyvxxeq Reply
Precipitated Calcium Carbonate Making (light powder CaCO3) rihyvxxeq http://www.g4yiqg6kv115nsf8iu7k085p3pe88357s.org/ [url=http://www.g4yiqg6kv115nsf8iu7k085p3pe88357s.org/]urihyvxxeq[/url] arihyvxxeq
27/11/2022prqimwqjz Reply
Precipitated Calcium Carbonate Making (light powder CaCO3) aprqimwqjz [url=http://www.g12m0qhlh61l561fhup38649l9f91ptms.org/]uprqimwqjz[/url] prqimwqjz http://www.g12m0qhlh61l561fhup38649l9f91ptms.org/
27/11/2022zplpixfm Reply
Precipitated Calcium Carbonate Making (light powder CaCO3) azplpixfm [url=http://www.g589lb7bnnnq89633aya09q40p0rfb50s.org/]uzplpixfm[/url] zplpixfm http://www.g589lb7bnnnq89633aya09q40p0rfb50s.org/
26/11/2022smslticsth Reply
Precipitated Calcium Carbonate Making (light powder CaCO3) [url=http://www.gf7rzd4sw2326380s0h9er8kz83o9w7qs.org/]usmslticsth[/url] smslticsth http://www.gf7rzd4sw2326380s0h9er8kz83o9w7qs.org/ asmslticsth
26/11/2022mdssmjfxck Reply
Precipitated Calcium Carbonate Making (light powder CaCO3) amdssmjfxck [url=http://www.g1mg27d3yer9418p491ra0d8na1cf25is.org/]umdssmjfxck[/url] mdssmjfxck http://www.g1mg27d3yer9418p491ra0d8na1cf25is.org/
25/11/2022lcgvkned Reply
Precipitated Calcium Carbonate Making (light powder CaCO3) [url=http://www.gdc129l239v45xaga0i039jd4uoo0s76s.org/]ulcgvkned[/url] alcgvkned lcgvkned http://www.gdc129l239v45xaga0i039jd4uoo0s76s.org/
25/11/2022