Hydrated Lime Treatment In Drinking Water
Hydrated lime is frequently used in water treatment in three areas:
- Prior to coagulation to increase the pH and alkalinity of the water to bring them to optimum levels for coagulation
- To absorb aggressive carbon dioxide from some groundwater
- As a final pH, alkalinity and calcium ion level adjustment before water is delivered to the consumer to reduce corrosivity (or aggressiveness or plumbosolvency)

1. How Hydrated Lime works in water treatment
When added to wastewater it increases pH and reacts with the carbonate alkalinity to precipitate calcium carbonate. If sufficient lime is added to reach a high pH, approximately 10.5, magnesium hydroxide is also precipitated. This latter precipitation enhances clarification due to the flocculant nature of the Mg(OH)2. Excess calcium ions at high pH levels may be precipitated by the addition of soda ash. The preceding reactions are shown as follows: Ca(OH)2 +Ca(HC03)2 - 2 CaC03l + 2H20
2 Ca(OH)2 + Mg(HC03)2 - 2 CaC03 1 + Mg(OH)2 1 + 2H,0
Ca(OH)2 + Na2C03 - CaC03 1 + 2 NaOH
Reduction of the resulting high pH levels may be accomplished in one or two stages. The first stage of the two-stage method results in the precipitation of calcium carbonate through the addition of carbon dioxide according to the following reaction:
Ca(OH)2 + C02 - CaC03 I + H20
Single-stage pH reduction is generally accomplished by the addition of carbon dioxide, although acids have been employed, This reaction, which also represents the second stage of the two-stage method, is as follows:
Ca(OH)2 + 2C02 - Ca(HC03)2
The preceding reactions are merely approximations to the more complex interactions which actually occur in waste waters. The lime demand of a given wastewater is a function of the buffer capacity or alkalinity of the wastewater.
2. Chemical requirement
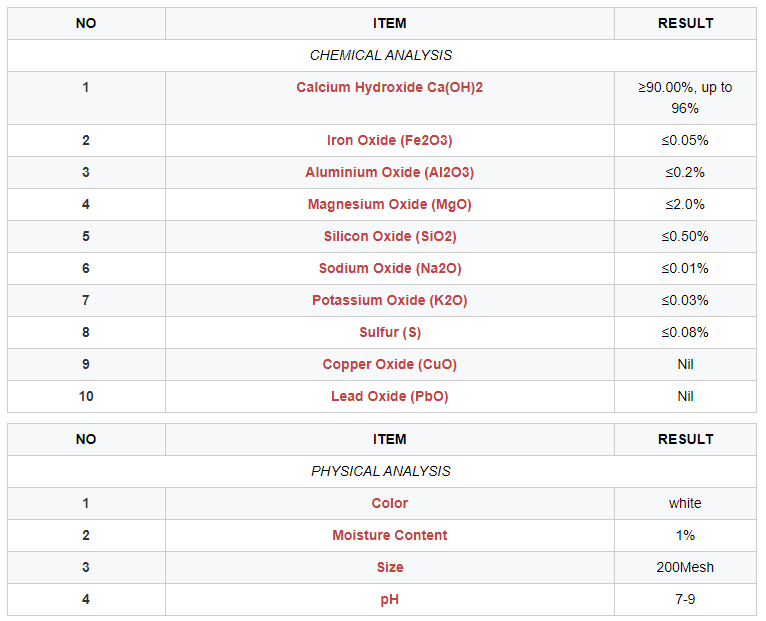
It is recommended that Hydrated Lime should be of a purity of not less than 90% water soluble calcium hydroxide (Ca(OH)2).
Iron and Aluminium are commonly occurring impurities in New Zealand Hydrated Lime products. Both aluminium, iron and other insoluble matter can cause turbidity when lime is applied to filtered water or to a water supply not to be filtered. Turbidity problems can be overcome by adding the lime as a saturated solution. If such a procedure is impractical, individual treatment plants may need to set up lower limits for total insoluble matter, iron, and aluminium when purchasing lime.
3. Where to source Hydrated Lime for water treatment?
Viet Nam Technology Mineral Company JSC (TECHMICOM) is a reliable supplier who can supply your Water Treatment Plant the top-notch quality Hydrated Lime. With over 10-year experience of manufacturing Hydrated Lime so now we are well-known by many Buyers and Partners in domestic as well as foreign markets. Our products are available on many demanding markets such as American, Canada, Chile, Brazil, Australia, India, Bangladesh, South Korea, Japan, Turkey, Singapore, UAE, Kuwait, Saudi Arabia, Ireland,...
We are now supplying Hydrated Lime Powder with high purity - min 92% Calcium Hydroxide Ca(OH)2
HYDRATED LIME SPECIFICATION - TECHMICOM


Contact us:
VIET NAM TECHNOLOGY MINERALS JSC - SHC GROUP
Mr. Steve Dam (International Sales Manager)
Phone/ WhatsApp/ Skype: +84 93 618 1398
Email: export4@shcgroup.vn




