Lime is a manufactured product made from limestone (calcium carbonate) or dolomite (calcium magnesium carbonate). The raw material is processed into quicklime and hydrated lime. Since it is alkaline, it's often used to adjust the pH of water and soils containing acidic components. It's used to treat both drinking water and wastewater.
Common Types of Lime Products for Water Treatment
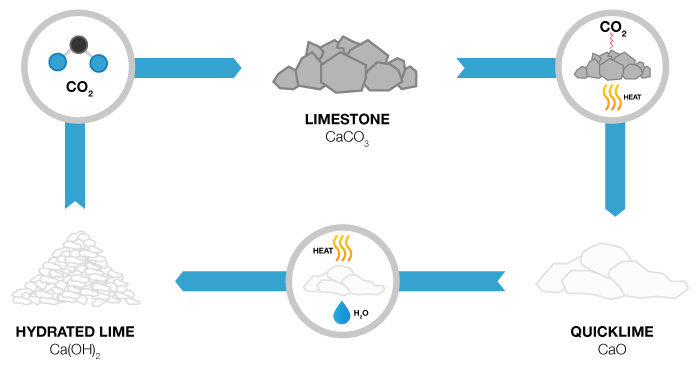
Plain calcium or magnesium carbonate is processed in several ways to create chemically different products for different purposes. Quicklime is made by heating calcium carbonate in a process called "calcining" to release carbon dioxide, leaving calcium oxide. Quicklime can be further processed by crushing and adding a small amount of water, to make hydrated lime, also called slaked lime, which is calcium hydroxide.
Lime in Water Softening
"Hard" water contains dissolved mineral compounds, including calcium and magnesium, and the softening process removes them. It might seem counterintuitive to add calcium to water in order to remove calcium from water, but the process uses chemical reactions in a high-pH environment to form calcium compounds that precipitate into solids, which can then be filtered out. For example, calcium bicarbonate reacts with lime to create calcium carbonate and water.
Lime in Municipal Sewage Treatment
As with water softening, lime raises the pH of sewage water containing phosphorus and nitrogen from organic sources, which can cause algae blooms. In the high-pH environment, lime combines with phosphorus to create calcium phosphates, which precipitate out of the water as a solid. "Ammonia stripping" uses that same high-pH environment to release nitrogen (as ammonium hydroxide) into the atmosphere, in the form of gas.
Lime in Industrial Wastewater Treatment
Many industrial processes -- from mining to steel making to fruit canning -- generate acidic wastewater, which must be treated before release. Lime serves to neutralize acids while also precipitating various metals into solids that can be recovered. Other, more caustic agents, such as caustic soda, could perform similar functions, but lime is cheaper and safer to handle, and its resulting sludge captures more metals with less tendency to leach them out.
If you have any question about Lime, please contact us via:
Telephone: +84 936 212 598
Hotline: +84 986 358 011 (Whatsapp / Wechat) - Mr. Thomas
Email: export2@shcgroup.vn
Skype: export2@shcgroup.vn




